Rapid Antigen Diagnostic Test Kit For Covid 19
Covid 19 rapid test kits have not been reviewed by the fda.
Rapid antigen diagnostic test kit for covid 19. This test has been authorized only for the detection of proteins from sarscov 2 not for any other viruses or pathogens. Covid 19 antigen rapid test covid 19 igm igg rapid test kit. The test has been authorized only for the detection of proteins from sars cov 2 not for any other viruses or pathogens and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and or diagnosis of covid 19 under section 564 b 1 of the act 21 u s c. Rapid test kits for coronavirus covid 19 should not be used as sole basis for diagnosis.
360bbb 3 b 1 unless. Most qpcr assays have three targets. The vstrip covid 19 antigen rapid test is intended for use by trained clinical laboratory personnel specifically instructed and trained in vitro diagnostic procedures. These may be molecular or antigen tests.
Following up with a molecular diagnostic testing should be considered to rule out infection in these individuals. The test kit is called the cdc 2019 novel coronavirus 2019 ncov real time reverse transcriptase rt pcr diagnostic panel. These include molecular tests and antigen tests. Therefore testing of covid 19 igm and igg antibodies is an effective method for the rapid diagnosis of covid 19 infection.
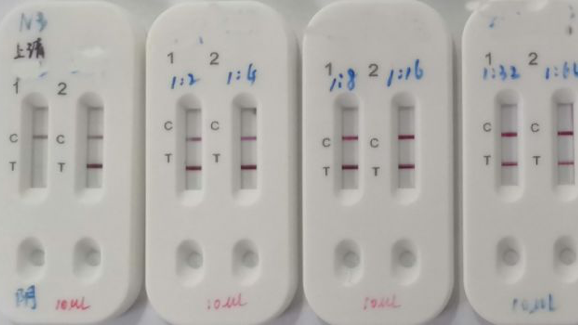
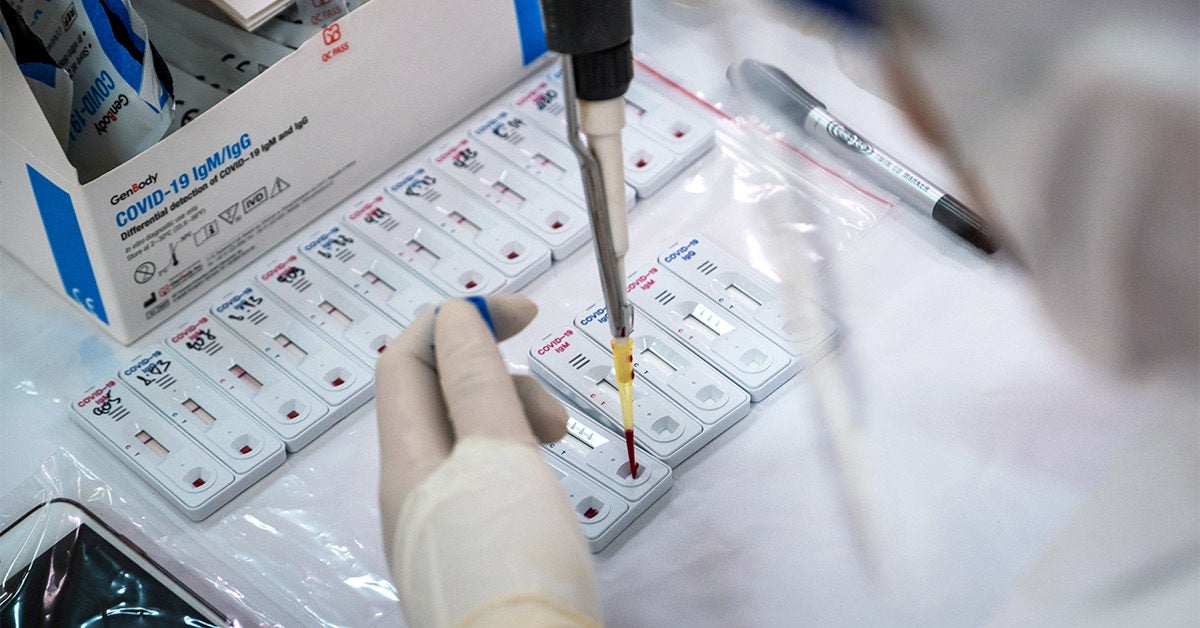
Furthermore detection of covid 19 igm antibodies tends to indicate a recent exposure to covid 19 whereas detection of covid 19 igg antibodies indicates a later stage of infection. Covid 19 antigen rapid swab test principle. Through our covid 19 diagnosis protocol medakit is able to combine antigenic with antibody testing that offers a comprehensive and highly accurate covid 19 diagnosis within 15 minutes. Rapid test kits for covid 19 point of care diagnostic test kits use a mucus sample from the nose throat or blood but can be analyzed at the doctor s office or clinic where the sample is collected and results may be available in minutes.
Orf1 e gene and n gene. Check what authorizations a kit has some kits are research use only ruo. This lateral flow assay is designed with the sandwich immunoassay format. Undefined covid 19 antigen rapid test kit fia uses advanced immunofluorescence based iateral flow technology in a sandwich design for qualitative detection of nucleocapsid protein from sars cov 2 covid 19 antigen rapid test kit fia with the fluorescent immuno analyzer model.
For in vitro diagnostic use. Negative results do not rule out sars cov 2 infection particularly in those who have been in contact with the virus. Afs 1000 provides automated and objective results in 15 minutes allowing for testing of patients suspected of.