Rapid Antigen Test Kit For Covid 19 Positive

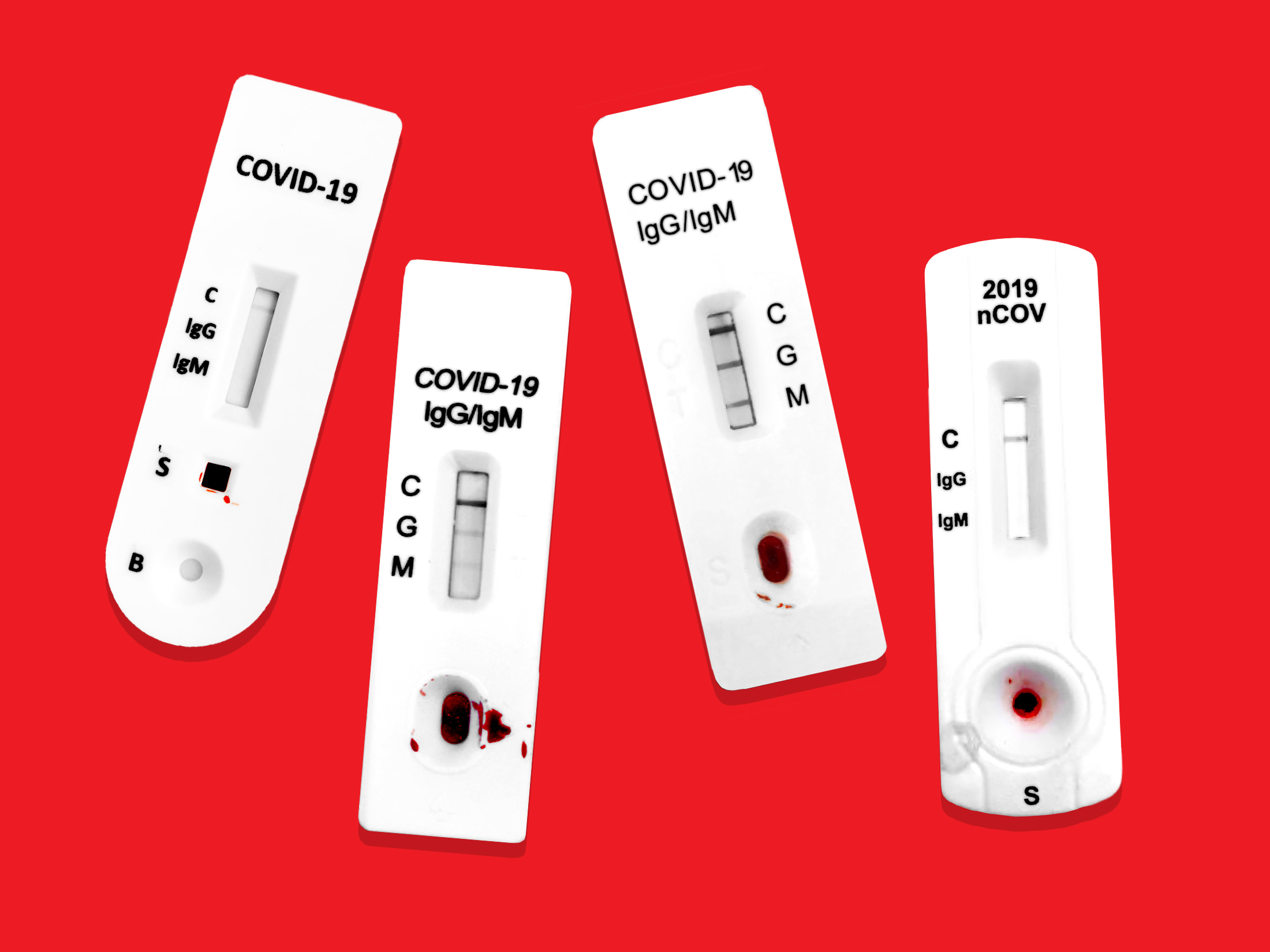
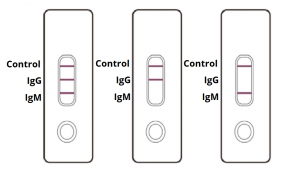
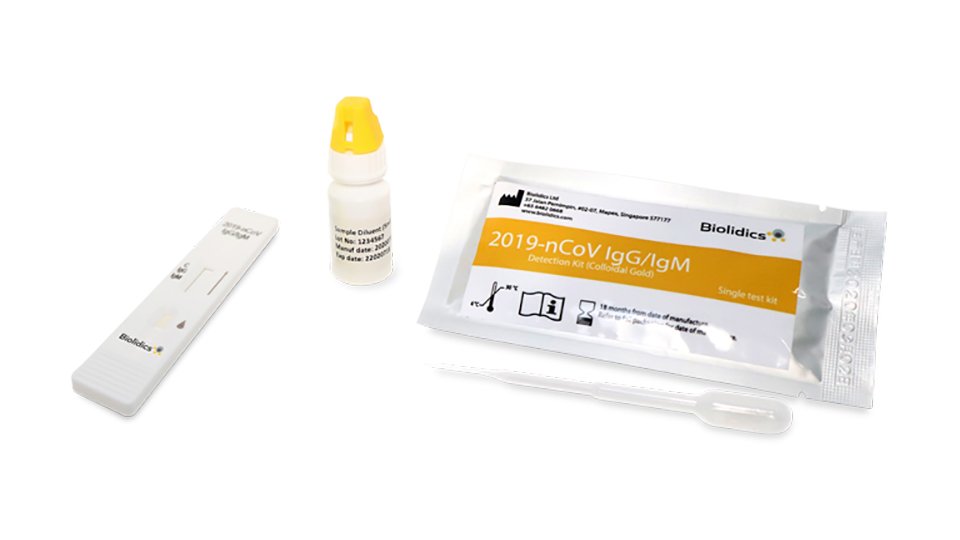
In response to the coronavirus covid 19 pandemic aurora is now offering the igm igg antibody rapid test kit to equip healthcare workers for rapid covid 19 antibody detection.
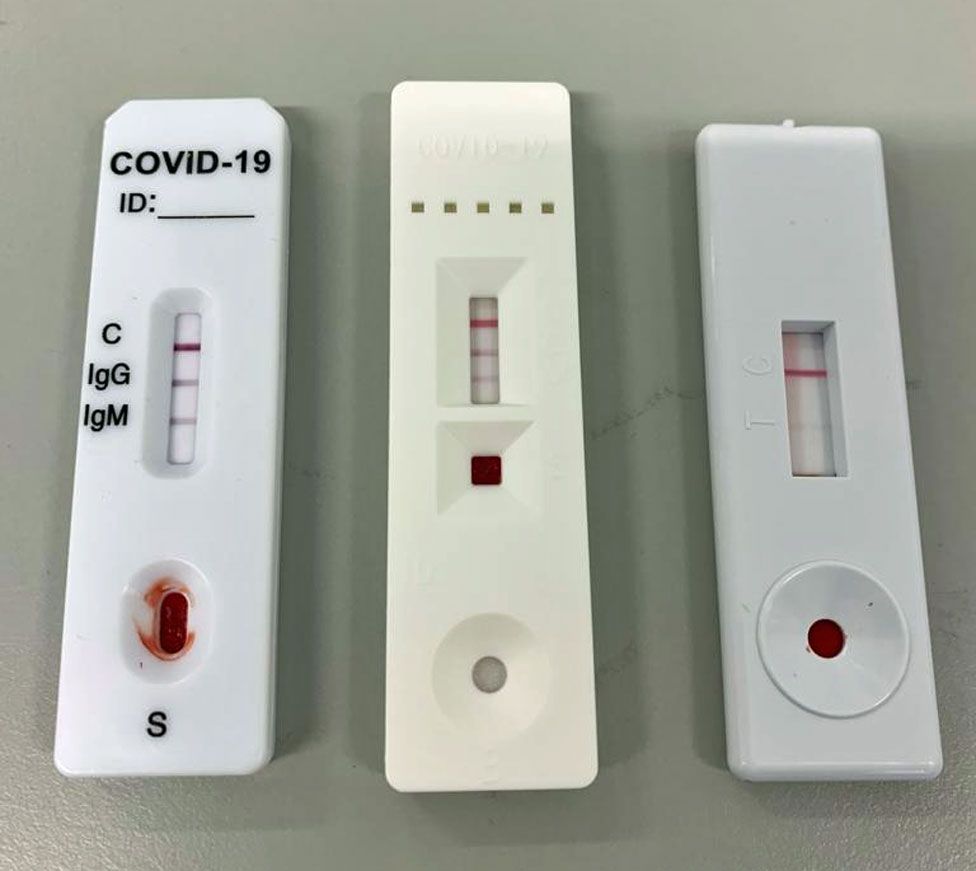
Rapid antigen test kit for covid 19 positive. Disregard test results beyond specified time 10 min. Medakit released the medakit covid 19 antigen rapid test a test that detects a covid 19 within 15 minutes. These include molecular tests and antigen tests. The post my covid 19 test was first positive.
The nids covid 19 antigen rapid test kit is intended for the in vitro qualitative detection of sars cov 2 virus antigen from sars cov 2 in nasopharyngeal and nasal swab specimens from individuals who are suspected of covid 19 by their healthcare provider. Do not interchange or mix different lots of vstrip covid 19 antigen rapid test. The coronavirus antigen rapid test kit is a lateral flow assay that qualitatively detects the presence of nucleocapsid n protein in upper respiratory samples nasal swabs. The sensitivity of current fda authorized antigen tests varies and thus negative diagnostic testing results should be handled differently depending on the testing device and its stated performance.
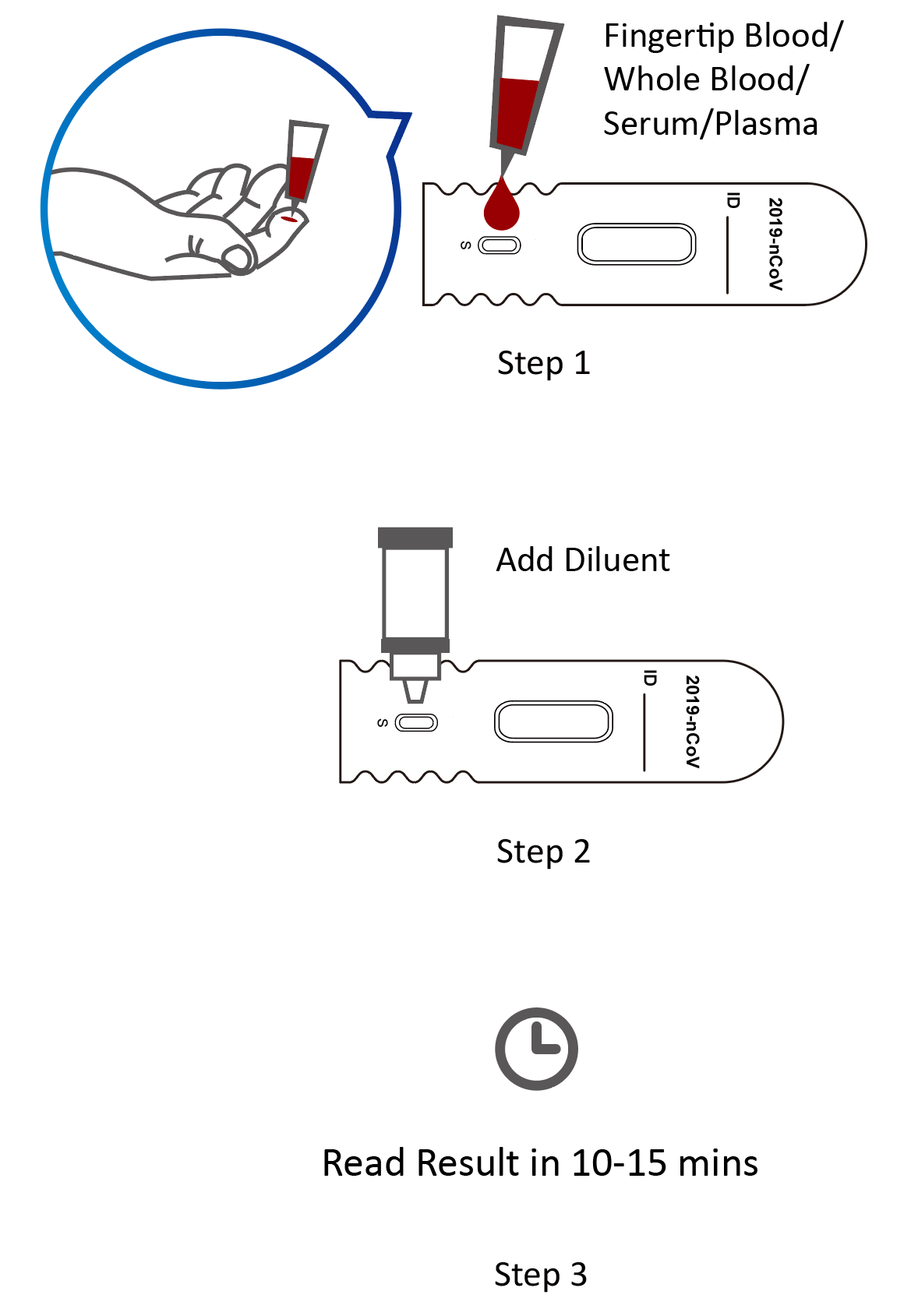
Rapid test kits for covid 19 point of care diagnostic test kits use a mucus sample from the nose throat or blood but can be analyzed at the doctor s office or clinic where the sample is collected and results may be available in minutes. A positive antigen test result is usually considered highly accurate. Covid 19 antigen rapid swab test principle. This lateral flow assay is designed with the sandwich immunoassay format.
October 15th 2020 medakit released the covid 19 rapid test kit for the novel coronavirus covid 19. Do not insert the test dipstick directly into the sampling area mouth nasal. Generally clinicians can rely upon a positive diagnostic antigen test result because the specificity of current fda authorized antigen tests is high in a person who has covid 19 symptoms. These may be molecular or antigen tests.
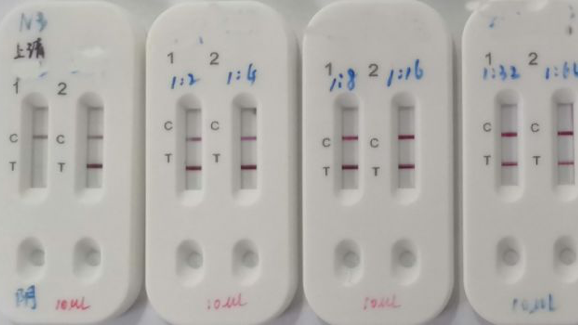
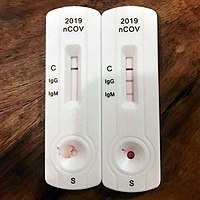
For the igm test 202 samples were tested positive out of 211 confirmed samples. Here s what it means and why it matters. Do not use the kit contents beyond the expiration date printed on the outside of the box.