Rapid Antigen Test Kit For Covid 19 Procedure

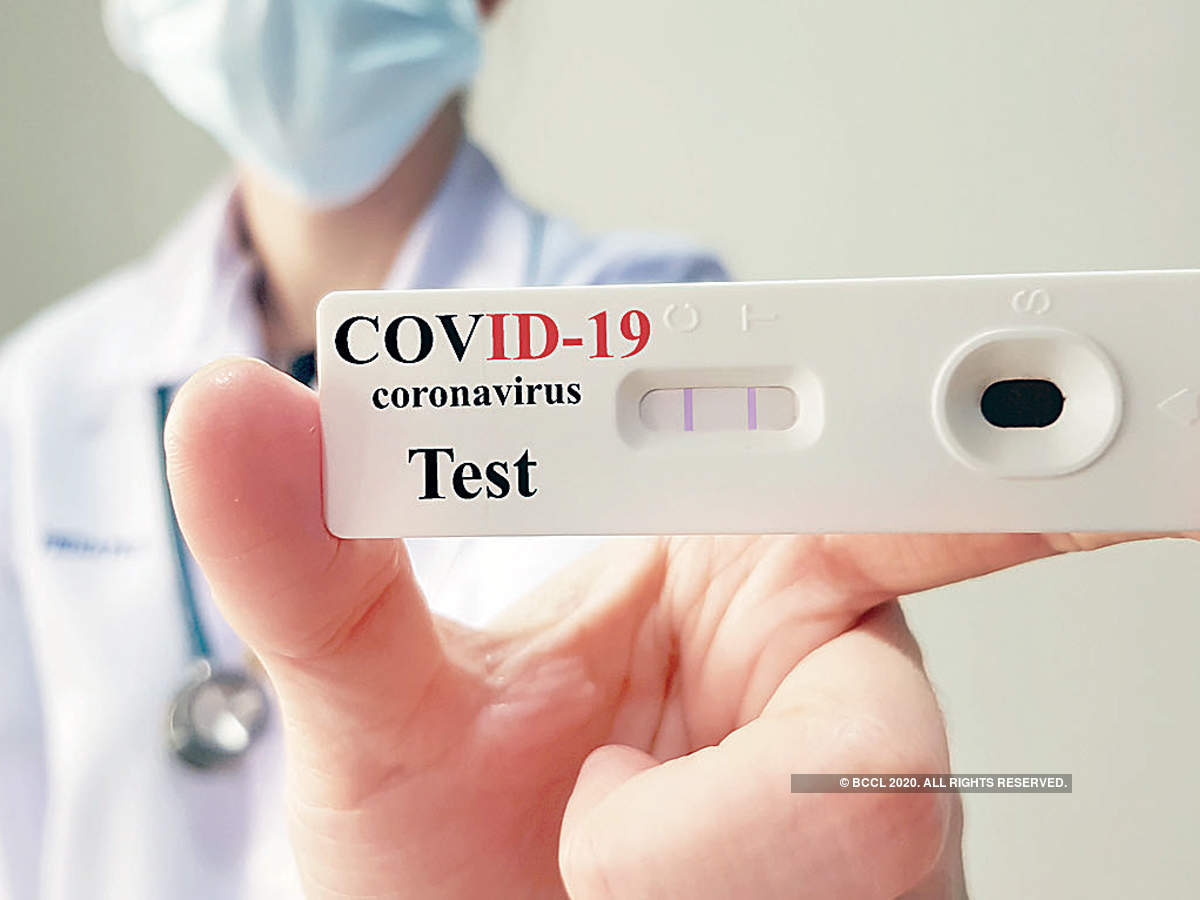
It is a test on swabbed nasal samples that detects antigens foreign substances that induce an immune response in the body that are found on or within the sars cov 2 virus.
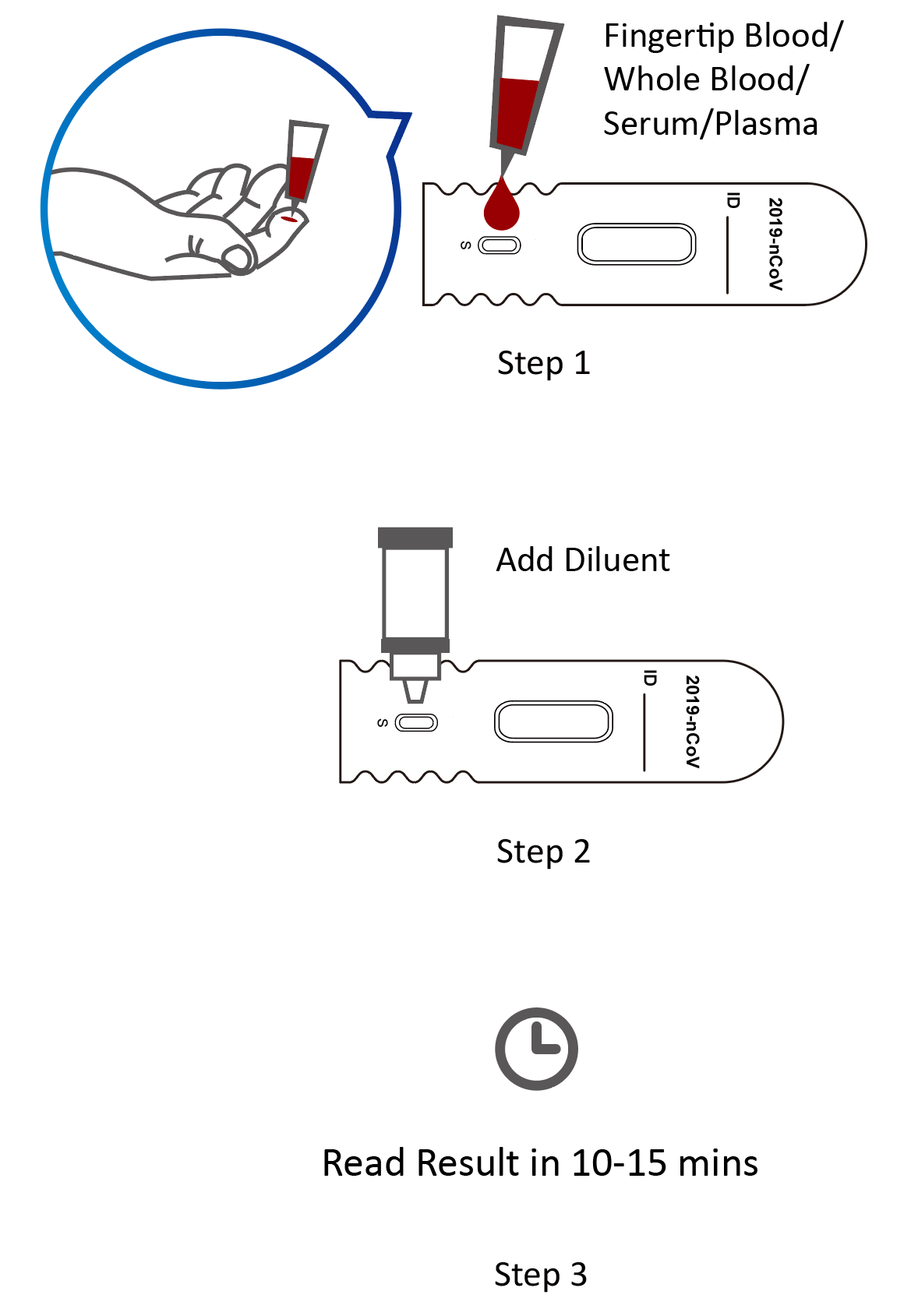
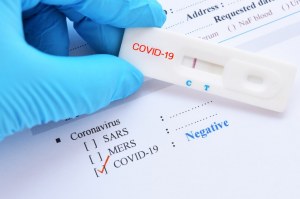
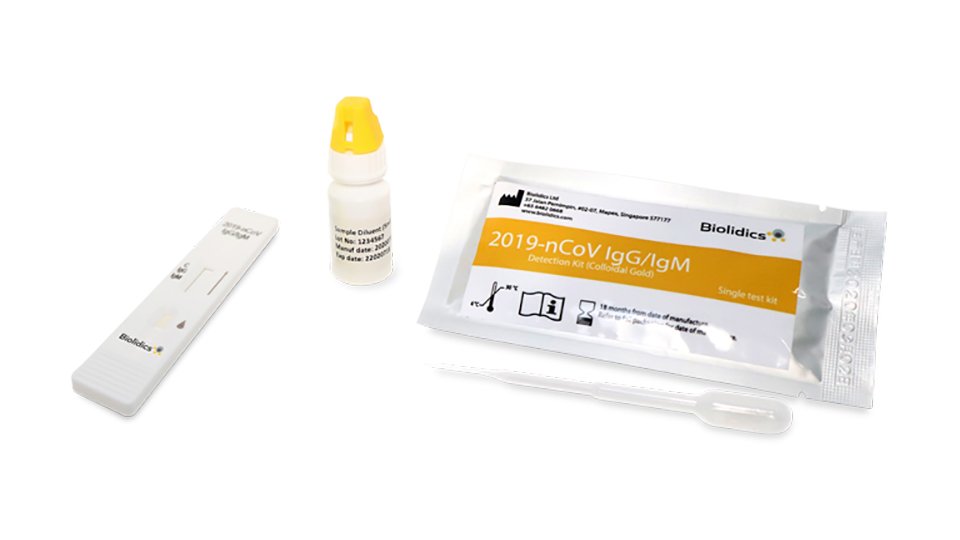
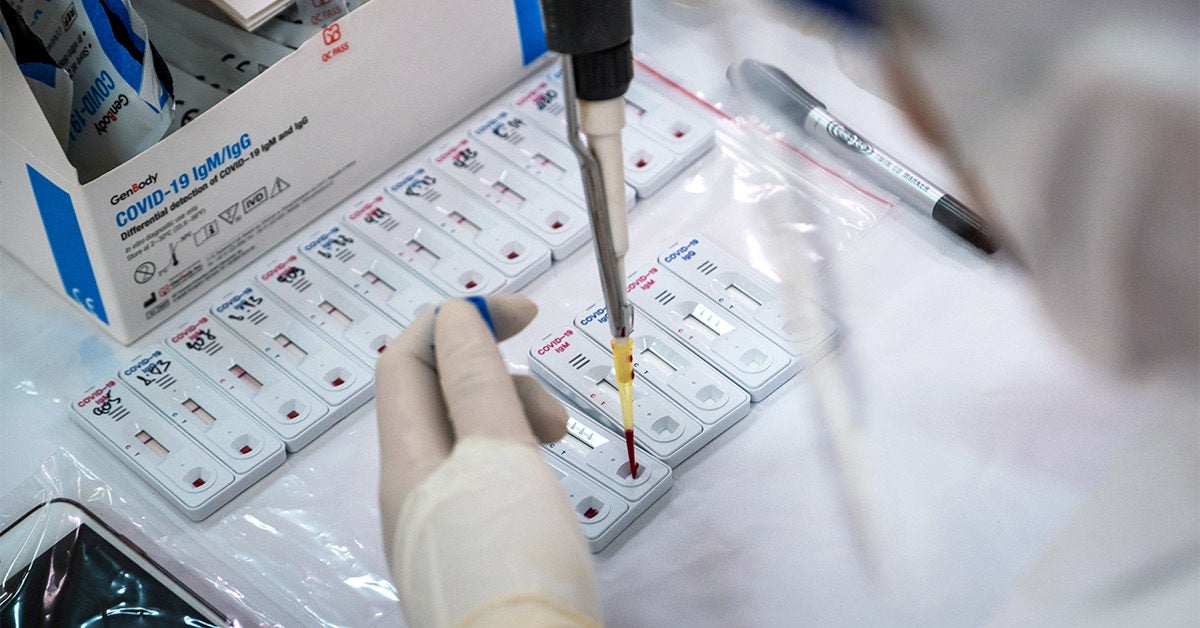
Rapid antigen test kit for covid 19 procedure. The rapid antigen detection test is to be used in specified settings and kits from only one manufacturer have got approval. Results should be used in combination with clinical observations and other testing methods such as nucleic acid pcr test. Rapid test kits for covid 19 point of care diagnostic test kits use a mucus sample from the nose throat or blood but can be analyzed at the doctor s office or clinic where the sample is collected and results may be available in minutes. Antigen tests can be used in a variety of testing strategies to respond to the coronavirus disease 2019 covid 19 pandemic.
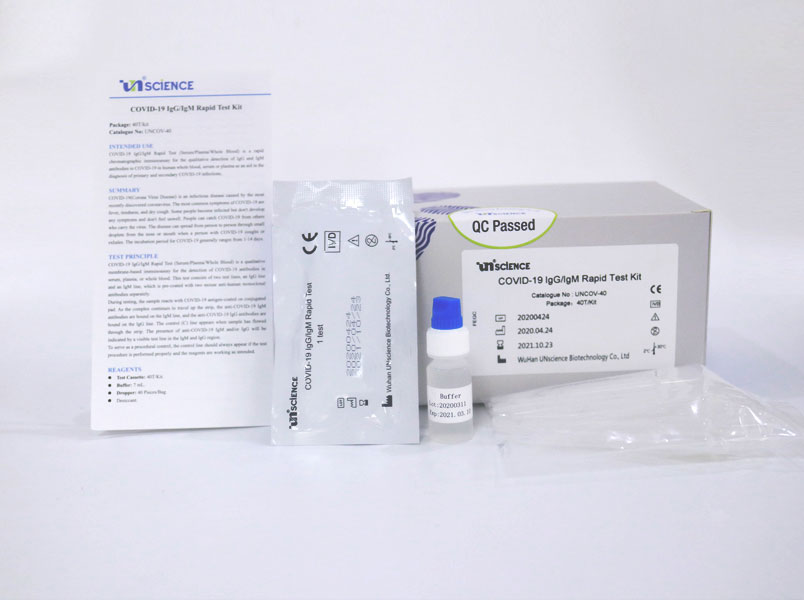
However results of test should not be the only basis for diagnosis. This interim guidance is intended for clinicians who order antigen tests receive antigen test results and or perform point of care testing as well as for laboratory professionals who perform antigen testing in a laboratory setting or at the point of care and. The nids covid 19 antigen rapid test kit is intended for the in vitro qualitative detection of sars cov 2 virus antigen from sars cov 2 in nasopharyngeal and nasal swab specimens from individuals who are suspected of covid 19 by their healthcare provider. This lateral flow assay is designed with the sandwich immunoassay format.
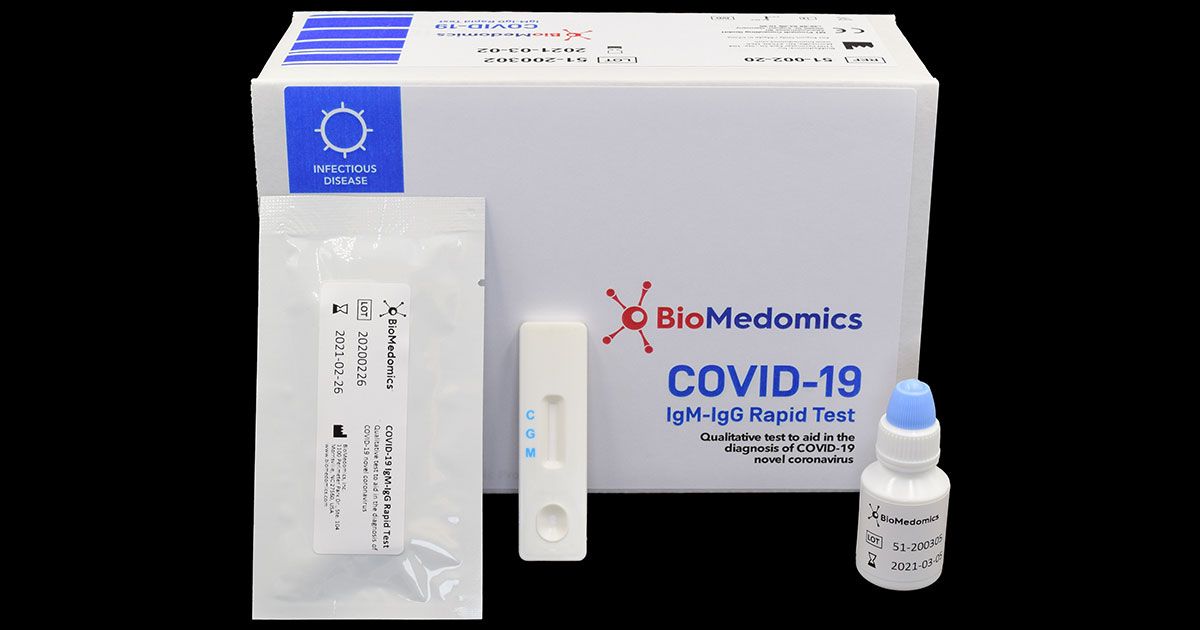
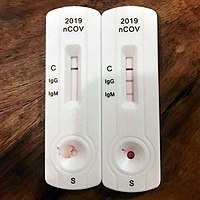
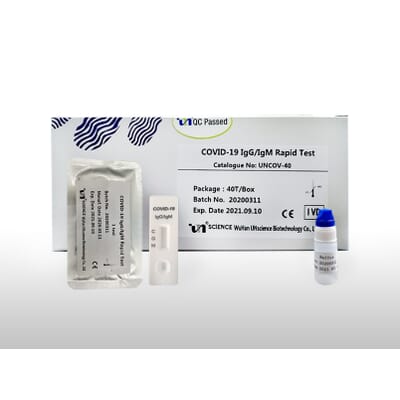
The vstrip covid 19 antigen rapid test is intended for use by trained clinical laboratory personnel specifically instructed and trained in vitro diagnostic procedures. These may be molecular or antigen tests. The covid 19 igm igg rapid testing kit can be used to screen patients suspected of having been affected by the novel coronavirus. The coronavirus antigen rapid test kit is a lateral flow assay that qualitatively detects the presence of nucleocapsid n protein in upper respiratory samples nasal swabs.
These include molecular tests and antigen tests. This test has been authorized only for the detection of proteins from sarscov 2 not for any other viruses or pathogens.