Rapid Antigen Test Kit For Covid 19
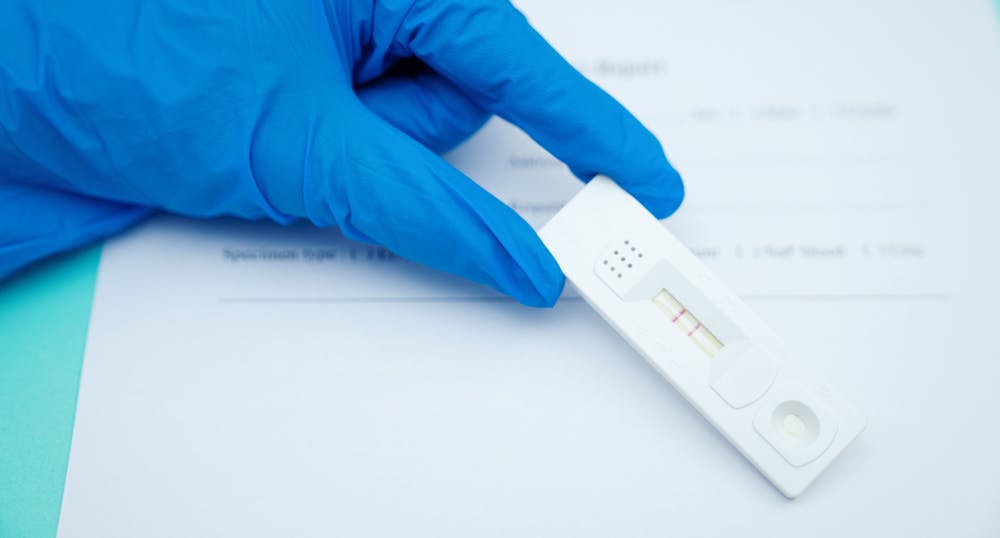
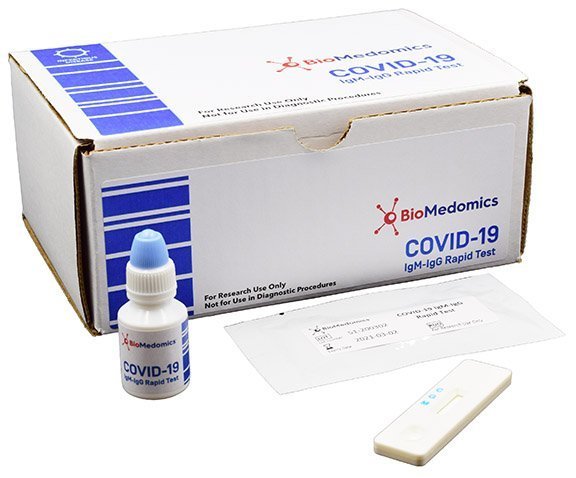
However results of test should not be the only basis for diagnosis.
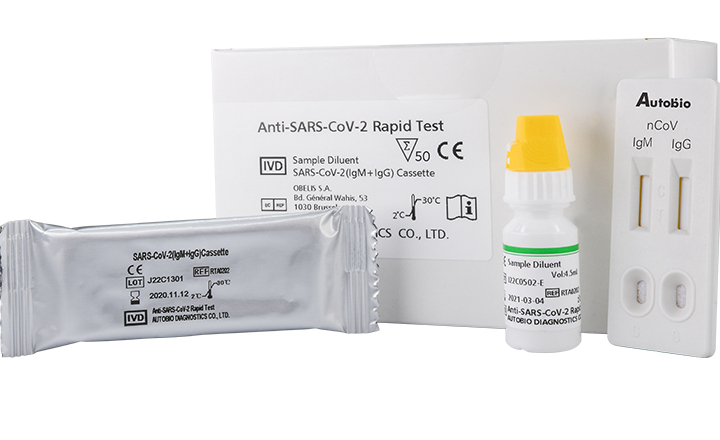
Rapid antigen test kit for covid 19. The nids covid 19 antigen rapid test kit is intended for the in vitro qualitative detection of sars cov 2 virus antigen from sars cov 2 in nasopharyngeal and nasal swab specimens from individuals who are suspected of covid 19 by their healthcare provider. To deal with the spread of the coronavirus disease 2019 covid 19 coris bioconcept has worked hard these past few weeks to develop a rapid antigen test that can be used for the detection of sars cov 2 in nasopharyngeal secretions. Carestart covid 19 antigen test allows for the rapid screening of covid 19 infection on a large scale. Orf1 e gene and n gene.
The coronavirus antigen rapid test kit is a lateral flow assay that qualitatively detects the presence of nucleocapsid n protein in upper respiratory samples nasal swabs. Check what authorizations a kit has some kits are research use only ruo manufacturers can apply for emergency use authorization eua from the us fda for clinical diagnostic use. Results should be used in combination with clinical observations and other testing methods such as nucleic acid pcr test. Increase your test capacities and stop the outbreak with an accurate antigen testing for severe and critical stages.
Covid 19 antigen rapid test. The rapid antigen detection test is to be used in specified settings and kits from only one manufacturer have got approval. Covid 19 antigen rapid swab test principle. These may be molecular or antigen tests.
What is the rapid antigen detection test for covid 19. 20 tests per box 29 50 per test. Most qpcr assays have three targets. These include molecular tests and antigen tests.
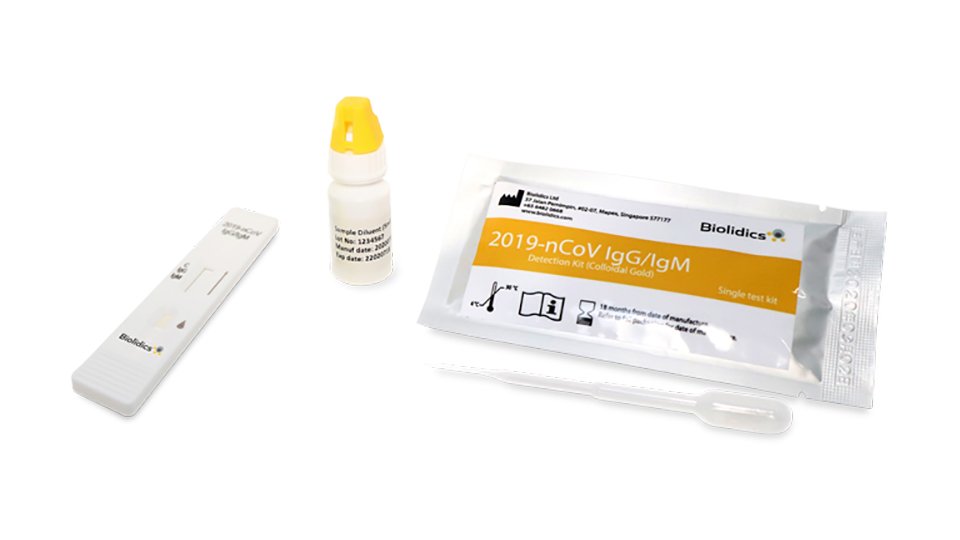
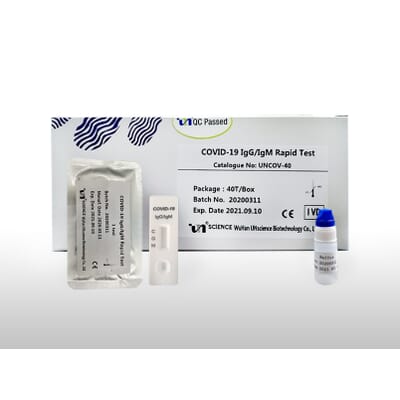
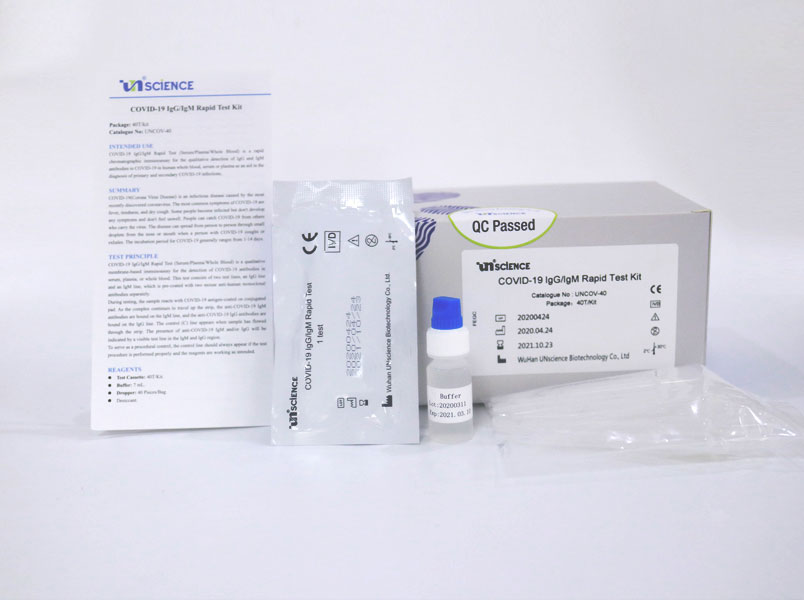
Rapid test kits for covid 19 point of care diagnostic test kits use a mucus sample from the nose throat or blood but can be analyzed at the doctor s office or clinic where the sample is collected and results may be available in minutes. The covid 19 igm igg rapid testing kit can be used to screen patients suspected of having been affected by the novel coronavirus. Rapid test kits for coronavirus covid 19 should not be used as sole basis for diagnosis.