Rapid Diagnostic Test Covid 19 Accuracy

This covid 19 test detects genetic material of the virus using a lab technique called polymerase chain reaction pcr.
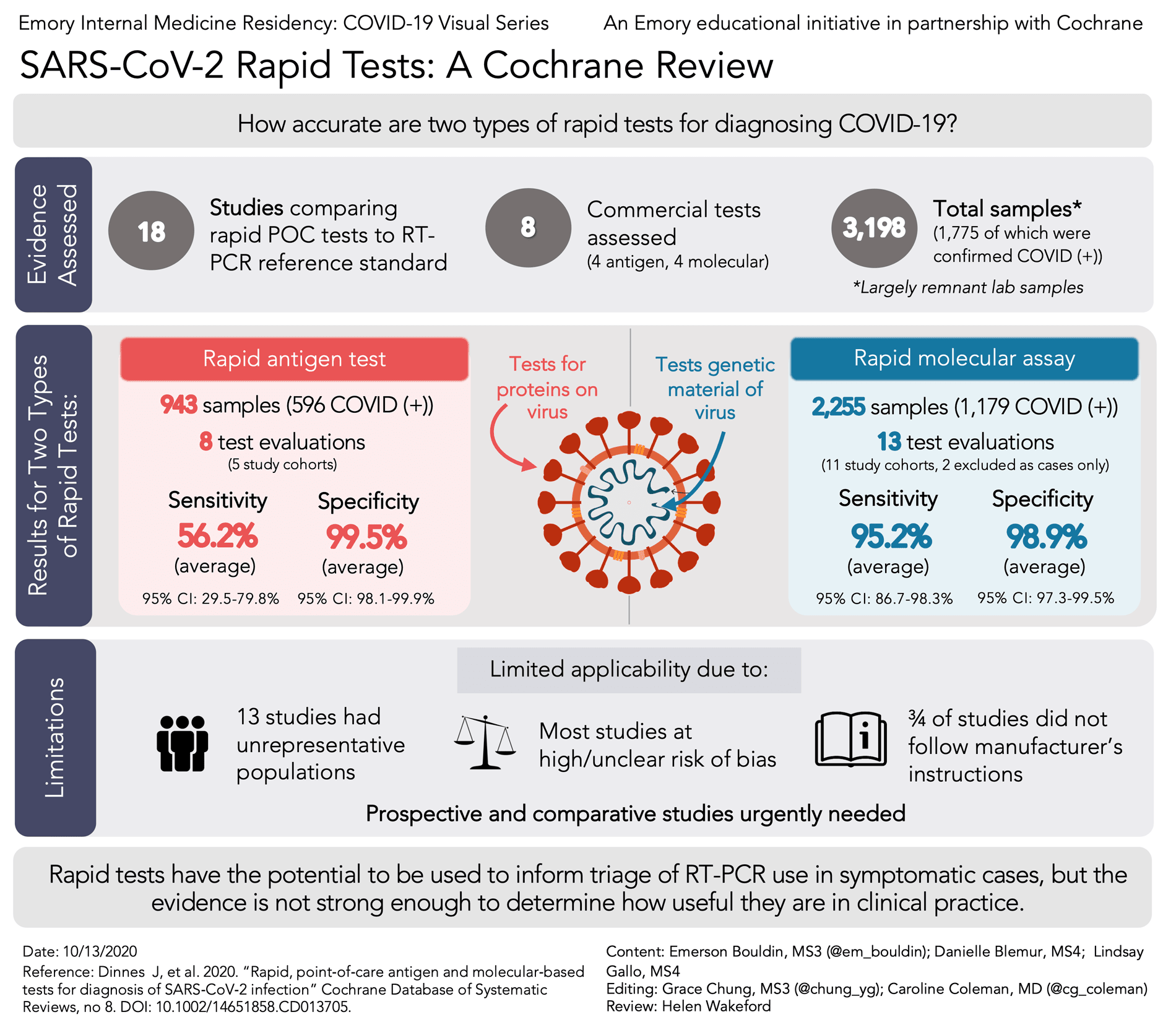
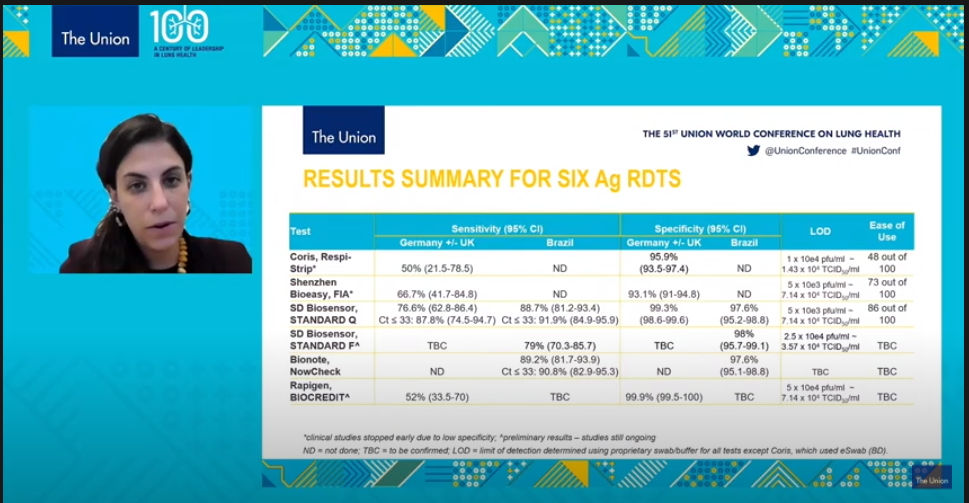
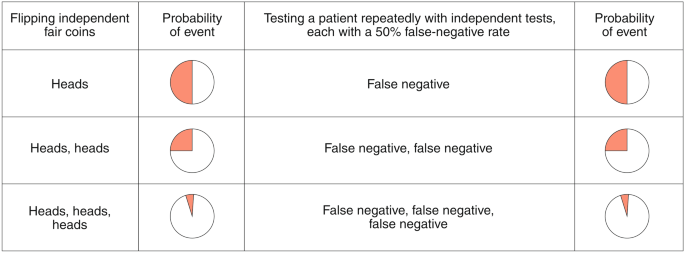
Rapid diagnostic test covid 19 accuracy. Cdc has developed two laboratory tests that identify sars cov 2 the virus that causes covid 19. Although rapid antigen detection tests radts for covid 19 provided a quicker turnaround than laboratory based diagnostics they are not as accurate as real time reverse transcription polymerase chain reaction rrt pcr tests the health watchdog has advised the national public health emergency team nphet. Wed oct 21 2020. Tests that are rapid usually providing results within 30 minutes are not as sensitive as the traditional test that is sent into the laboratory and generally takes half a day to several days to.
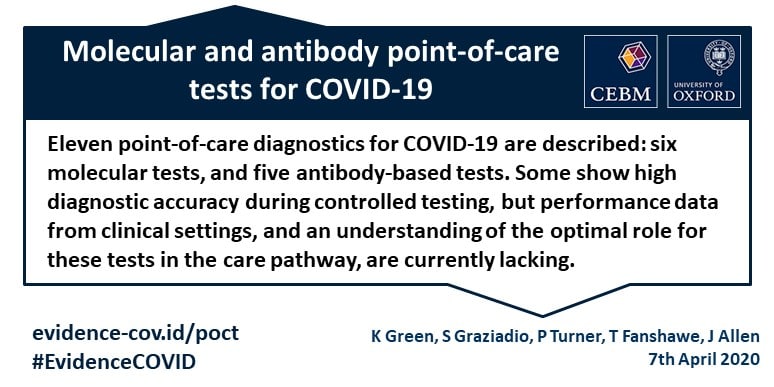
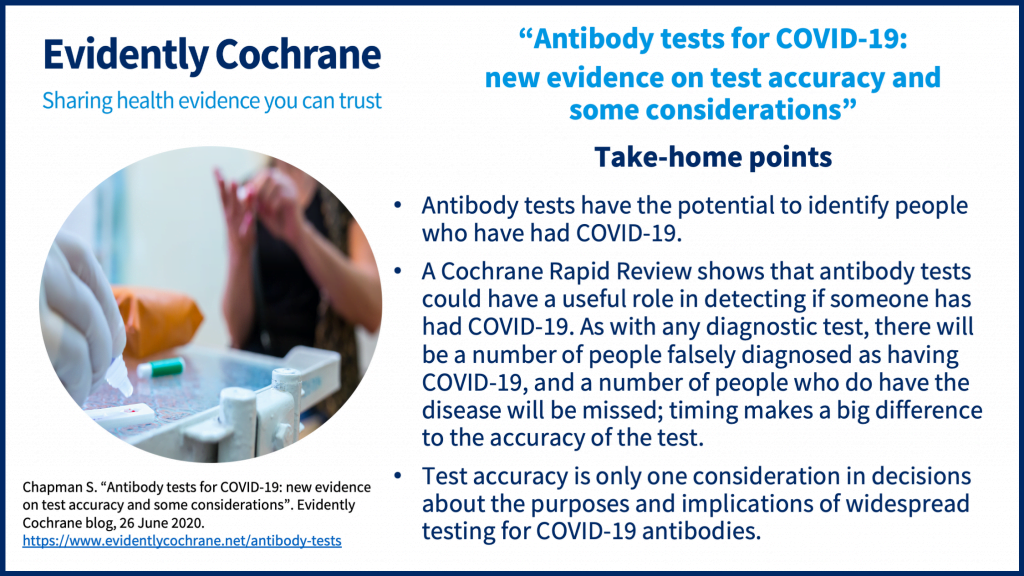
Any rapid antigen test for sars cov 2 authorized for use by fda will be included on fda s list of in vitro. Rapid tests are point of care diagnostic tests that use a mucus sample from the nose or throat but can be analyzed at a doctor s office or clinic instead of being. Testing for all three viruses at the same time will provide public health officials with information they need to help reduce the spread of these viruses in the community while conserving resources that are in short supply. The 3 types of covid 19 tests are a molecular pcr test antigen rapid test and an antibody blood test.
What is a rapid covid 19 test. Also called a molecular test a health care worker collects fluid from a nasal or throat swab or from saliva. Find out how each test is performed and how accurate they are. Covid 19 assays and test systems used for diagnostic or screening testing including those for antigen testing must have received an eua from fda or be offered under the policies in fda s policy for covid 19 tests external icon.
Rapid tests have reduced diagnostic accuracy hiqa says cheaper tests should be considered but none could be recommended as yet wed oct 21 2020 10 00 updated. Results may be available in minutes if analyzed onsite or a few days or longer in locations with test processing delays if sent to an outside lab.