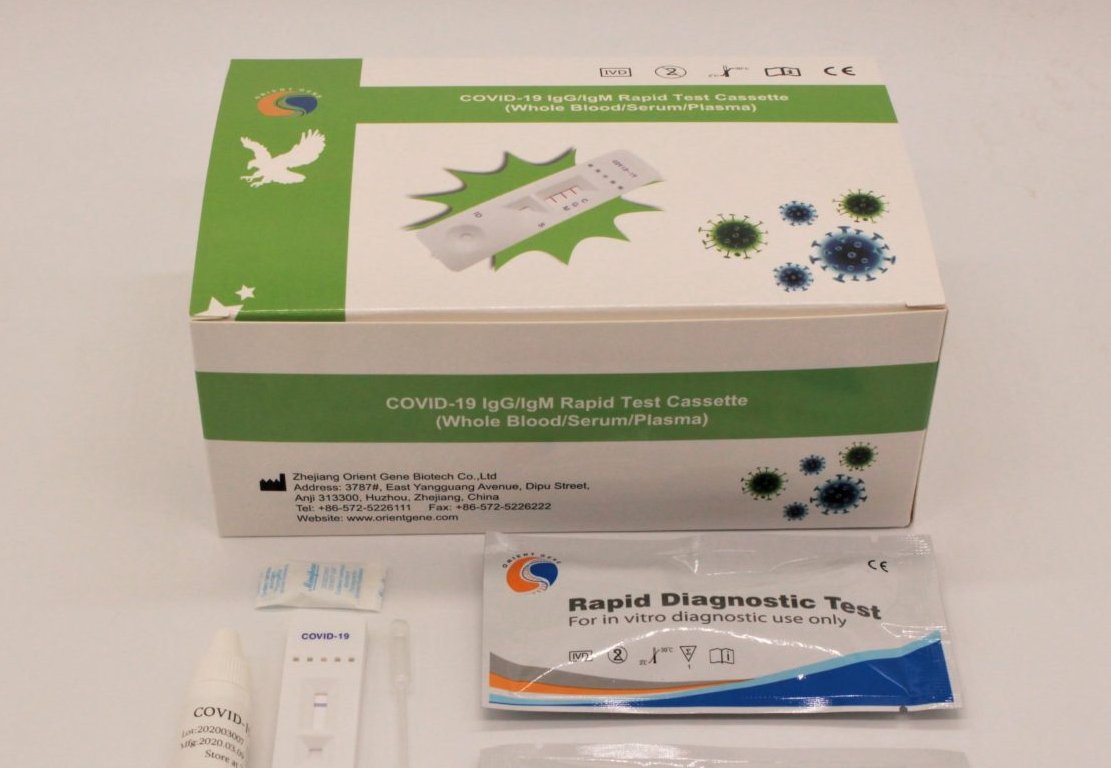
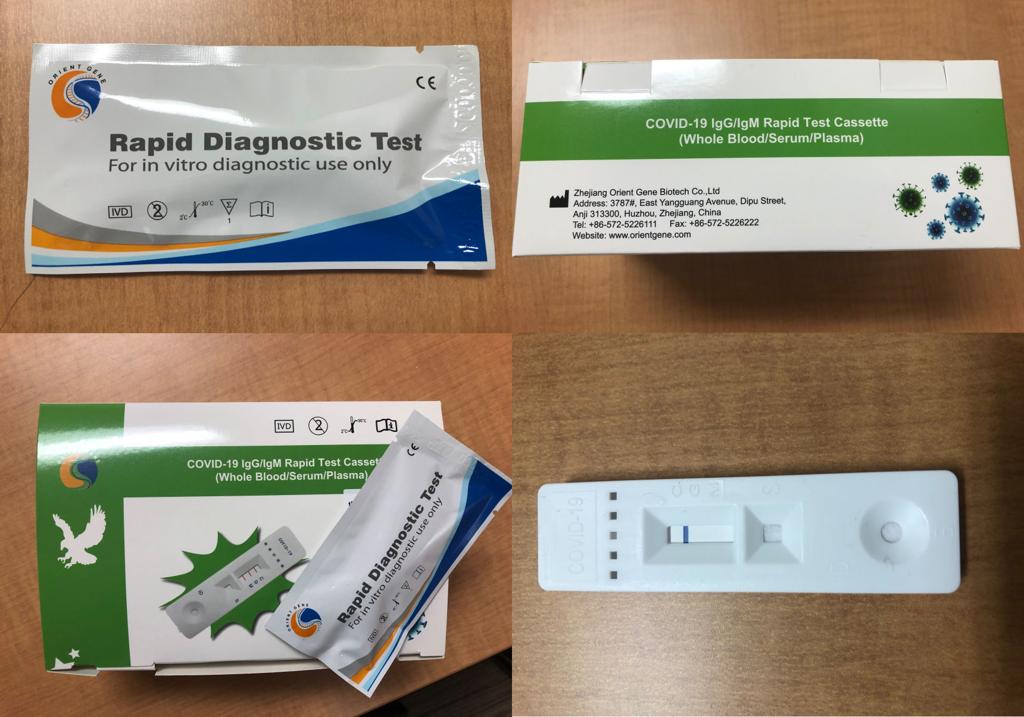
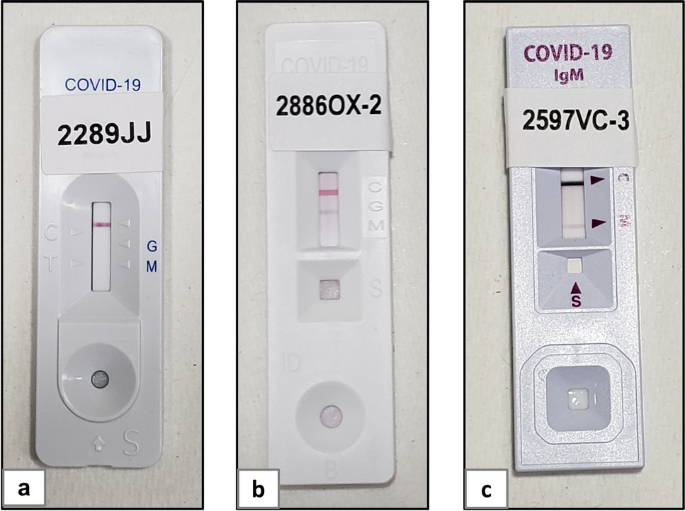
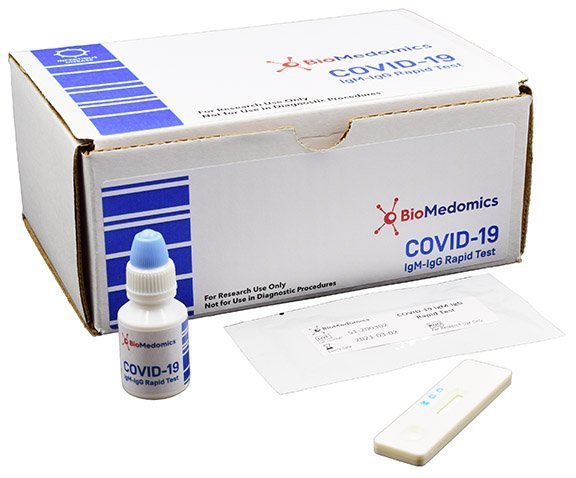
Rapid Diagnostic Test Covid 19 Orient Gene
In general antibodies can be detected 1 3 weeks after infection.
Rapid diagnostic test covid 19 orient gene. E gene s gene orf1ab gene rdrp gene key reagents. Here we report performance characteristics of orient gene biotech covid 19 igg igm rapid test cassette og and compare it to abbott sars cov 2 igg. Covid 19 the coronavirus ag rapid test cassette swab is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from sars cov 2 in nasopharyngeal np swab specimens directly or after the swabs have been added to viral transport media from individuals who are suspected of covid 19 by their healthcare provider. Here we report performance characteristics of orient gene biotech covid 19 igg igm rapid test cassette og and compare it to abbott sars cov 2 igg immunoassay asia.
The rapid test is. These point of care test kits are registered for use in countries with reliable regulatory agencies such as china and singapore. In stock fast delivery easy to perform inexpensive method to test immunology safe to use the covid 19 igg igm rapid test is valididated fda and tga aproved and has a ce marking. Global regulators have largely stepped out of diagnostics manufacturers way to enable them to quickly bring covid 19 tests to the public.
Covid 19 diagnostics in context csb nucleic acid tests nats for viral rna most common targets. Orient gene covid 19 lgg lgm rapid test instructions. Cdc approved kits include 2019 ncov cdc probe and primer kit for sar2 cov 2 biosearch technologies and 2019 ncov kit idt. While the coronavirus disease 2019 covid 19 pandemic has peaked in many countries already the current challenge is to assess population immunity on a large scale.
This test is intended to screen patients for sars cov 2 antibodies. The orient gene covid 19 igg igm rapid test detects if there are antibodies present agains the coronavirus. Many serological tests are available and require urgent independent validation. Many serological tests are available and require urgent independent validation.
N1 n2 genes single or multiple other emerging targets. Press statement 30 march 2020 the food and drug administration fda approves the use of five 5 rapid test kits for covid 19 today march 30 2020. Viral genome sequence mn908947 cdc approved targets. Colloidal gold rapid diagnostic test.
Healgen scientific danwei biotechnology. The fda supports all efforts to address this pandemic.