Rapid Diagnostic Test Covid 19
The test kit is called the cdc 2019 novel coronavirus 2019 ncov real time reverse transcriptase rt pcr diagnostic panel.
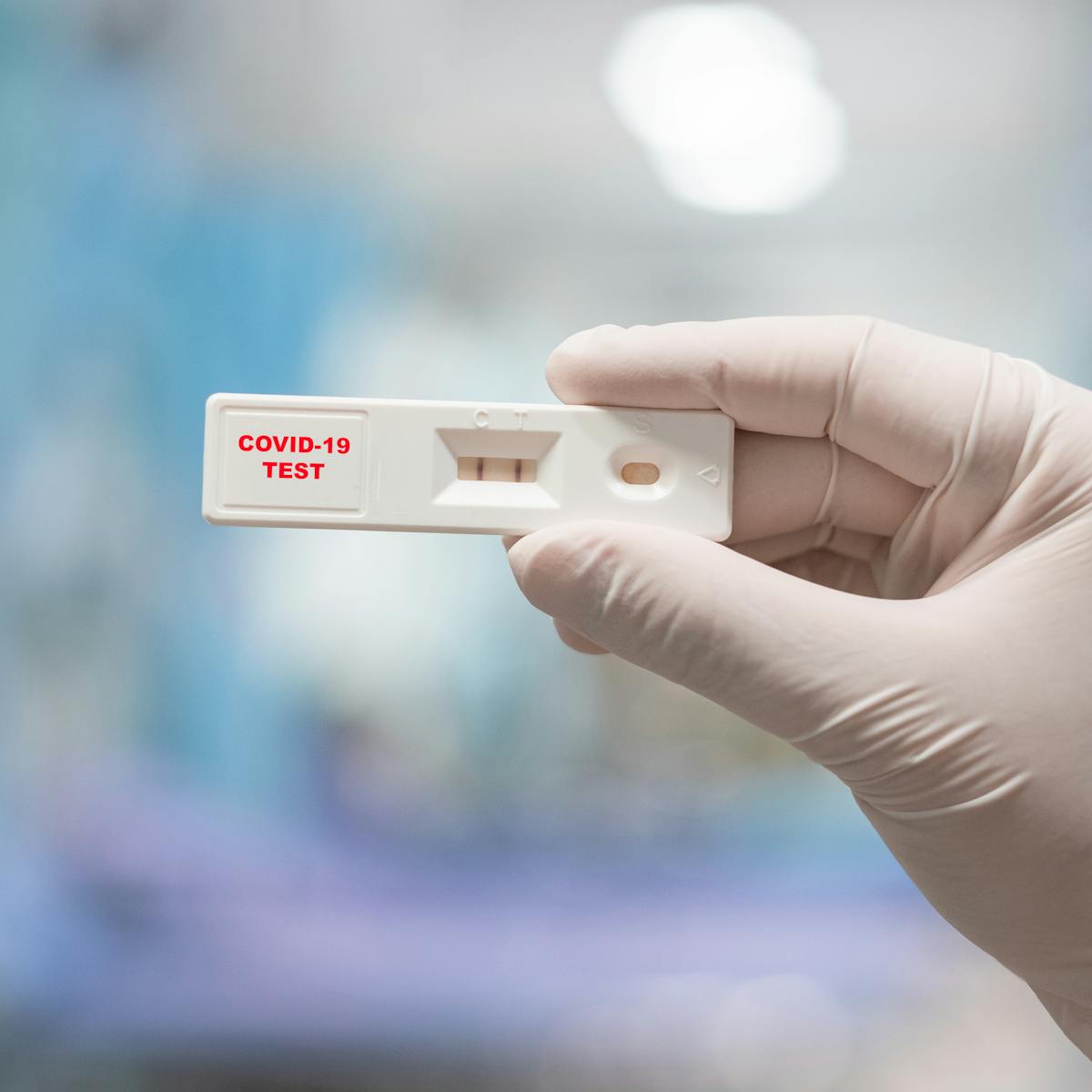
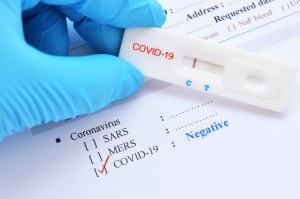
Rapid diagnostic test covid 19. Tests for viral presence are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Covid 19 assays and test systems used for diagnostic or screening testing including those for antigen testing must have received an eua from fda or be offered under the policies in fda s policy for covid 19 tests external icon. You can test yourself easily at home and have the results within 20 minutes. Certain fda authorized covid 19 test kits allow you to collect the sample at home and then send it to a lab to be tested though a sample collected by a trained professional and sent to a certified lab is.
In early 2020 cdc developed its first laboratory test kit for use in testing patient specimens for sars cov 2. What is a rapid covid 19 test. Our interactive map tracks the number of sars cov 2 tests reported to have been performed in each country and compares it with the number that showed a positive result to build a picture of how many people in the world are being tested for covid 19. Rapid tests are point of care diagnostic tests that use a mucus sample from the nose or throat but can be analyzed at a doctor s office or clinic instead of being.
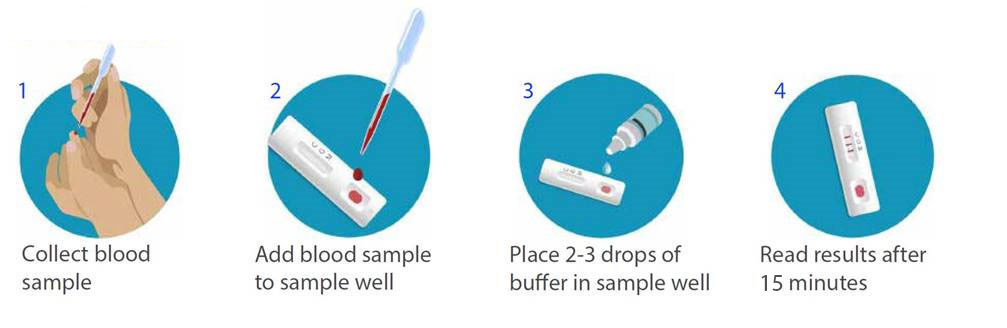
Complete instructions in english are included in the kit. For igm and 21 days i e. The covid 19 rapid diagnostic test rdt can only be used on people who had onset of symptoms for at least 5 days i e. This is the same type of test that was used to detect severe acute respiratory.
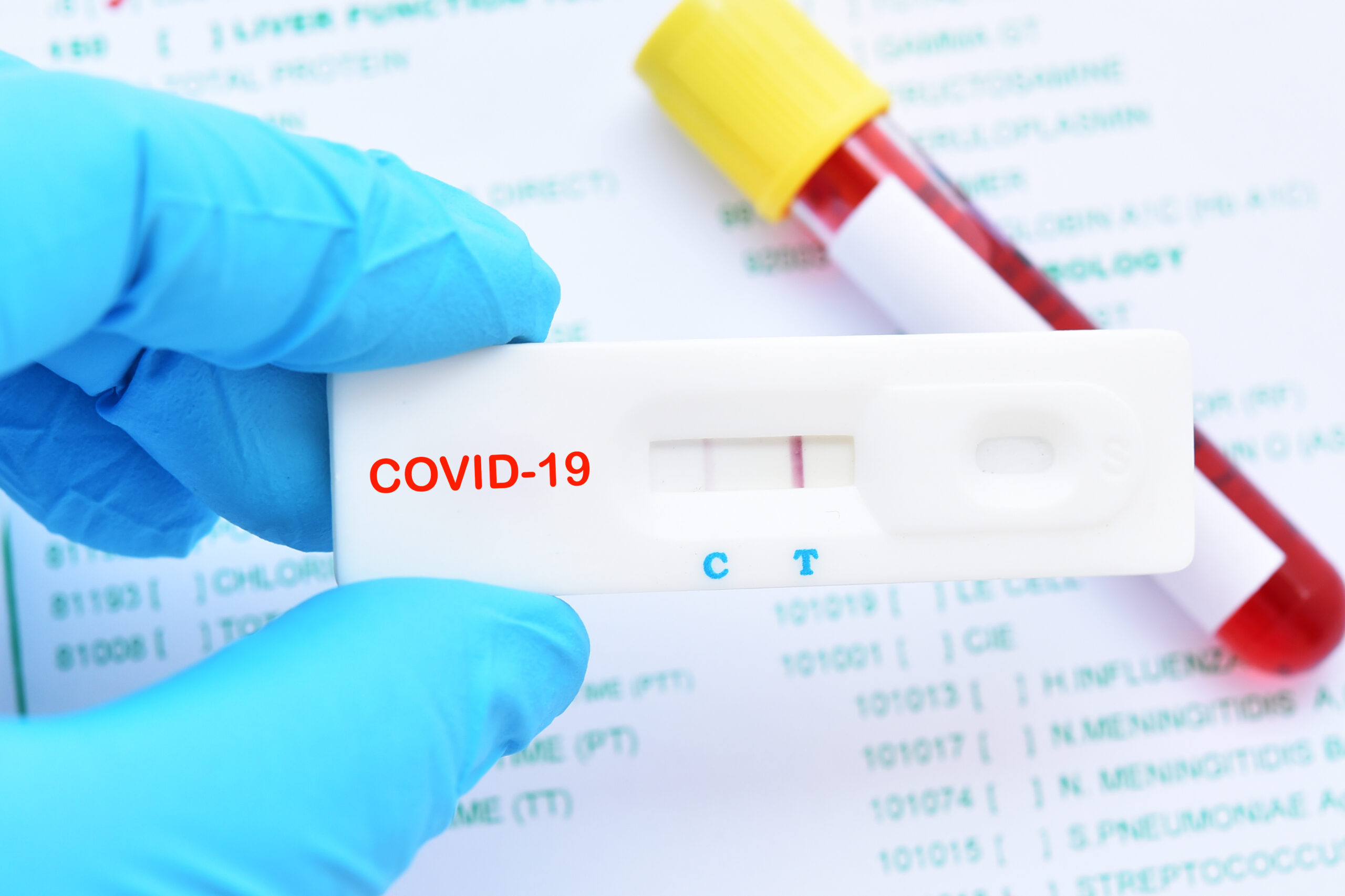
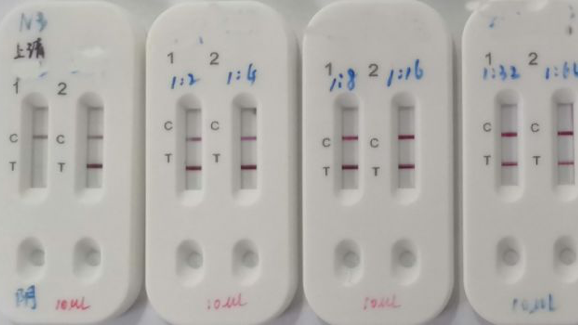
Current testing situation worldwide. Rapid diagnostic tests for covid 19. Covid 19 testing involves analyzing samples to assess the current or past presence of sars cov 2 the two main branches detect either the presence of the virus or of antibodies produced in response to infection. Most kits include both igm and igg so.
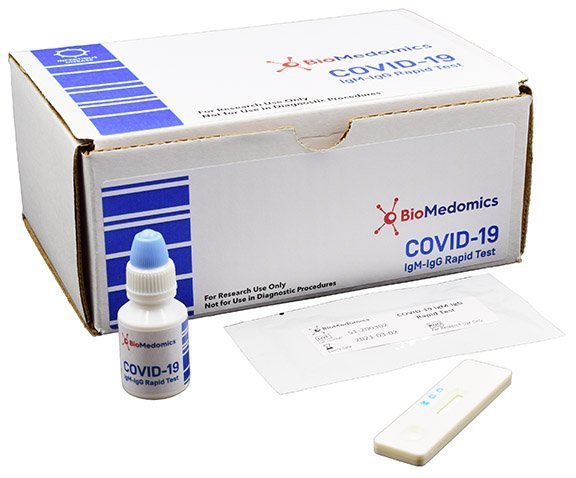
On february 3 2020 cdc submitted an eua package to expedite fda permitted use of the cdc diagnostic panel in the united states. This video shows how to use covid 19 igm igg rapid test kit. Any rapid antigen test for sars cov 2 authorized for use by fda will be included on fda s list of in vitro. This is not for home use.
.jpg)