Rapid Test Covid 19 Abbott

To help fight the coronavirus pandemic.
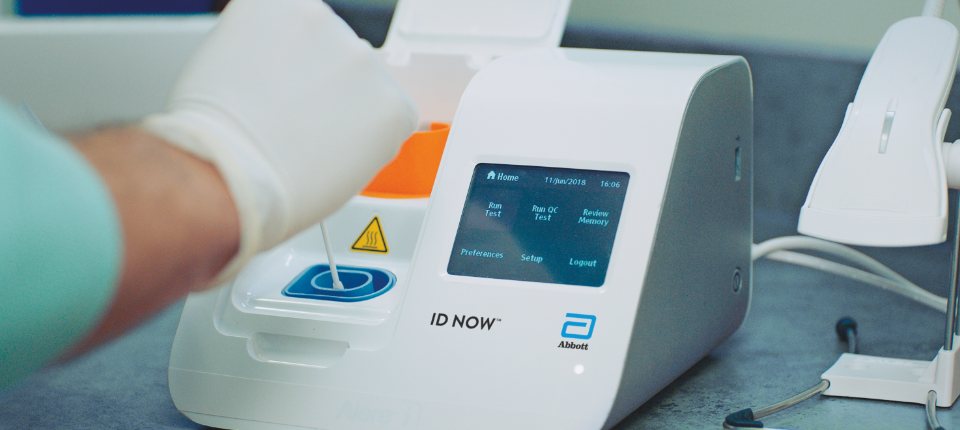
Rapid test covid 19 abbott. For patients suspected of current covid 19 infection. Id now instruments were already placed throughout the u s. Interim data from abbott s 1 003 participant study shows that its test which can deliver results in under 15 minutes correctly identified positive covid 19 cases 95 of the time when used. Abbott labs got emergency approval from the us food and drug administration for its rapid antigen test which can detect a covid 19 infection in 15 minutes.
Abbott has currently developed six covid 19 tests three are molecular they detect the virus during active infection one is an antigen test identifying covid 19 mid infection as the virus multiplies and two are serology tests they identify igg antibodies proteins the body produces in the late stages of infection which may remain in the blood for months and possibly longer after a. The binaxnow covid 19 ag card is the sixth test that abbott is launching in the u s. The id now covid 19 rapid test delivers high quality molecular positive results in as little as 5 minutes targeting the coronavirus covid 19 rdrp gene. Interim data from abbott s 1 003 participant study shows that its test which can deliver results in under 15 minutes correctly identified positive covid 19 cases 95 of the time when used within seven days of symptom onset.
Food and drug administration emergency use authorization eua. Rapid molecular or rt pcr tests include the id now by abbott cobas by roche cue covid 19 test by cue health xpert xpress by cepheid and accula by mesa biotech. The id now covid 19 assay is now available for use on the id now platform under u s. And being used to test for the flu and strep.
Food and drug administration issued an emergency use authorization. When we saw the seriousness of covid 19 we knew we needed to develop all types of testing that could help curb the spread and support care for patients said chris scoggins senior vice president rapid diagnostics abbott. Requires no instrumentation and provides results in 15 minutes making it a valuable tool for mass testing in decentralized settings. Abbott s tests are performed on its high volume m 2000 and alinity m molecular laboratory systems.
Wainer executive vice president of abbott s rapid and molecular diagnostics businesses said. Its id now rapid molecular point of care platform.