Rapid Diagnostic Test Covid 19 How To Use
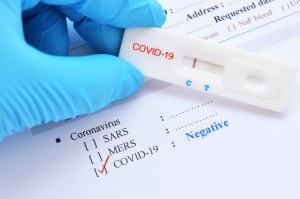
There is another more common type of rapid diagnostic test marketed for covid 19.
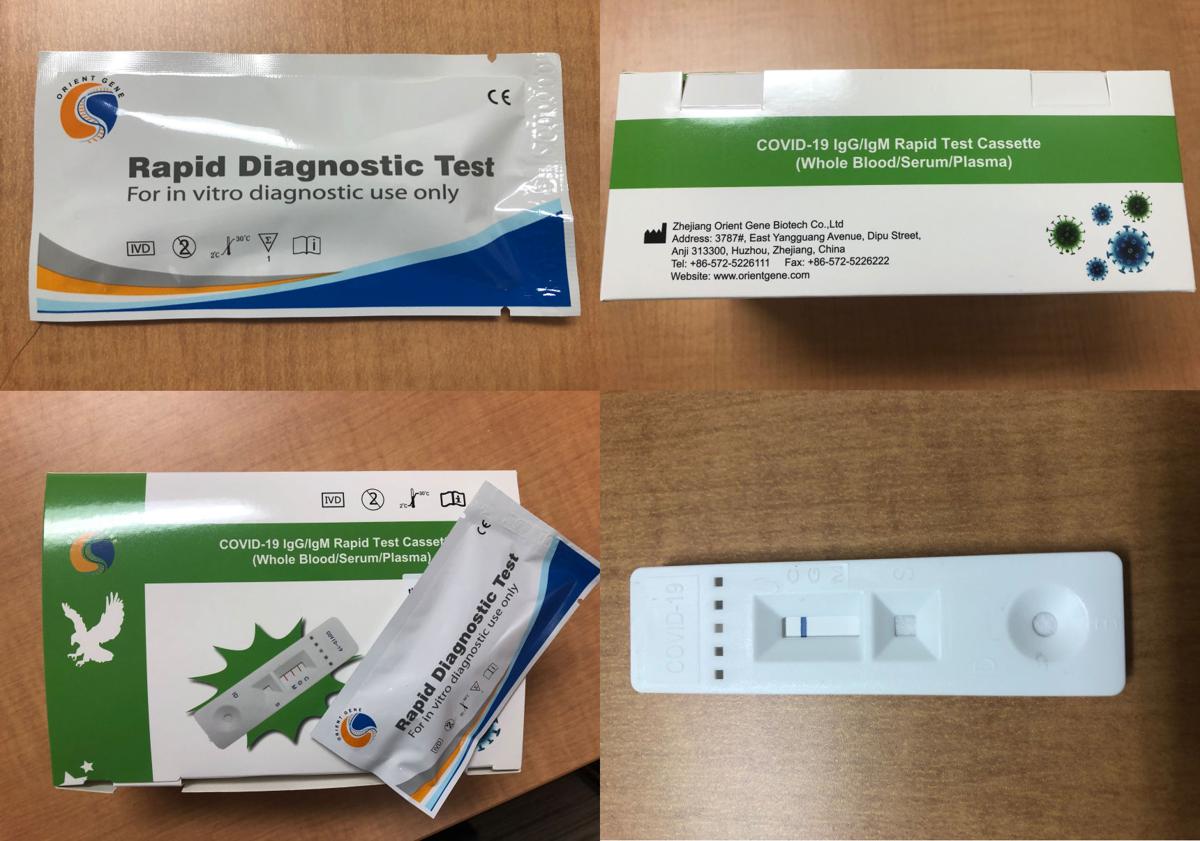
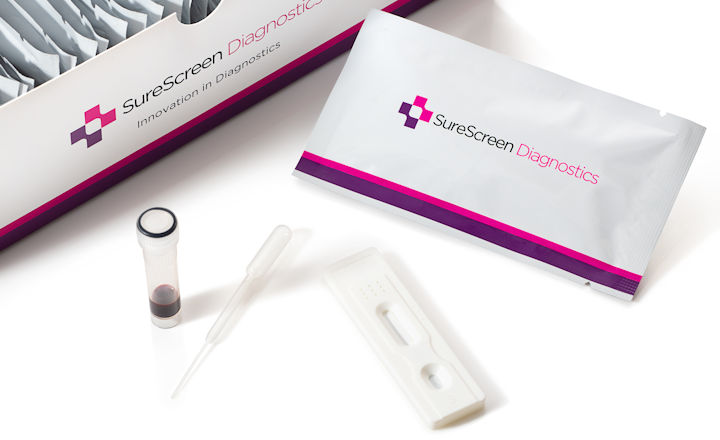
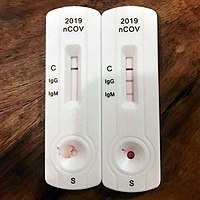
Rapid diagnostic test covid 19 how to use. This is a rapid test for covid 19 and it will determine if you are contaminated with covid 19 or you have already suffered from covid 19 in the past based on igg and igm antibodies. On february 3 2020 cdc submitted an eua package to expedite fda permitted use of the cdc diagnostic panel in the united states. This covid 19 test detects genetic material of the virus using a lab technique called polymerase chain reaction pcr. Covid 19 diagnosis as it can take 10 days or more after onset of symptoms for patients to become positive for detectable antibodies 3 4 and because the antibodies persist long after the infection has cleared.
Complete instructions in english are included in the kit. In early 2020 cdc developed its first laboratory test kit for use in testing patient specimens for sars cov 2. Results may be available in minutes if analyzed onsite or a few days or longer in locations with test processing delays if sent to an outside lab. The test kit is called the cdc 2019 novel coronavirus 2019 ncov real time reverse transcriptase rt pcr diagnostic panel.
Also called a molecular test a health care worker collects fluid from a nasal or throat swab or from saliva. Rapid tests are point of care diagnostic tests that use a mucus sample from the nose or throat but can be analyzed at a doctor s office or clinic instead of being. Rapid diagnostic tests based on host antibody detection. The fda recently authorized the use of a rapid coronavirus diagnostic test as part of its efforts to expand screening capacity.
The fda approved point of care poc testing devices made by. You can test yourself easily at home and have the results within 20 minutes. Any rapid antigen test for sars cov 2 authorized for use by fda will be included on fda s list of in vitro. For igm and 21 days i e.
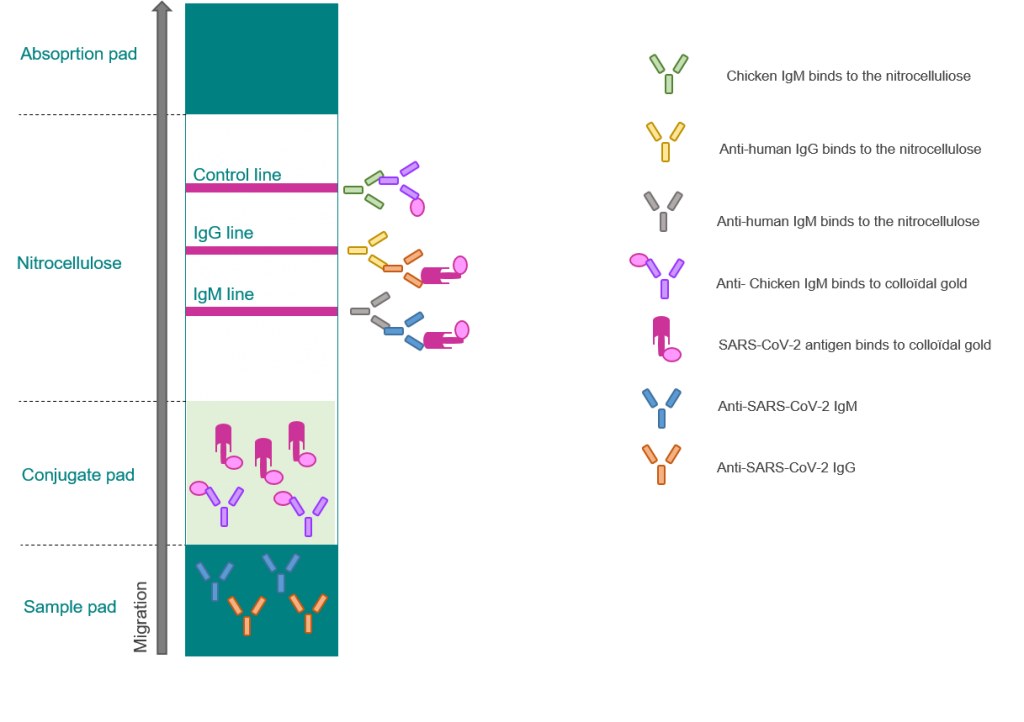
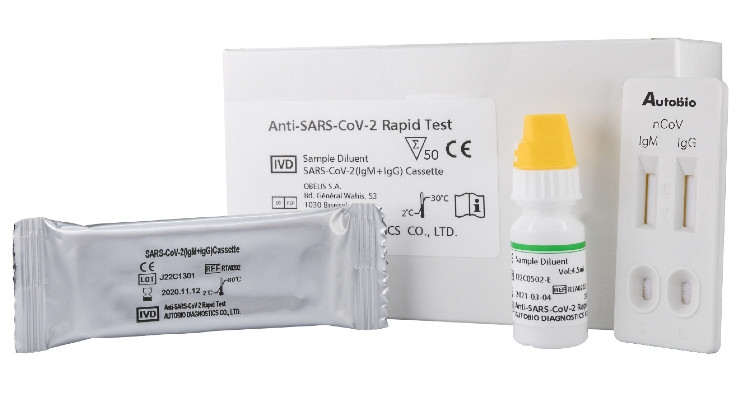
A test that detects the presence of antibodies in the blood of people believed to have been infected with covid 19. Covid 19 assays and test systems used for diagnostic or screening testing including those for antigen testing must have received an eua from fda or be offered under the policies in fda s policy for covid 19 tests external icon. The covid 19 rapid diagnostic test rdt can only be used on people who had onset of symptoms for at least 5 days i e. Most kits include both igm and igg so.
What is a rapid covid 19 test. Genetic or molecular tests antigen tests and antibody tests. An increase in rapid testing could help stop the spread of covid 19.