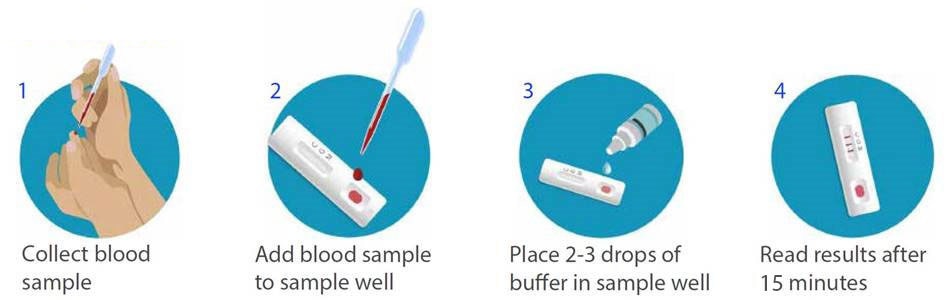
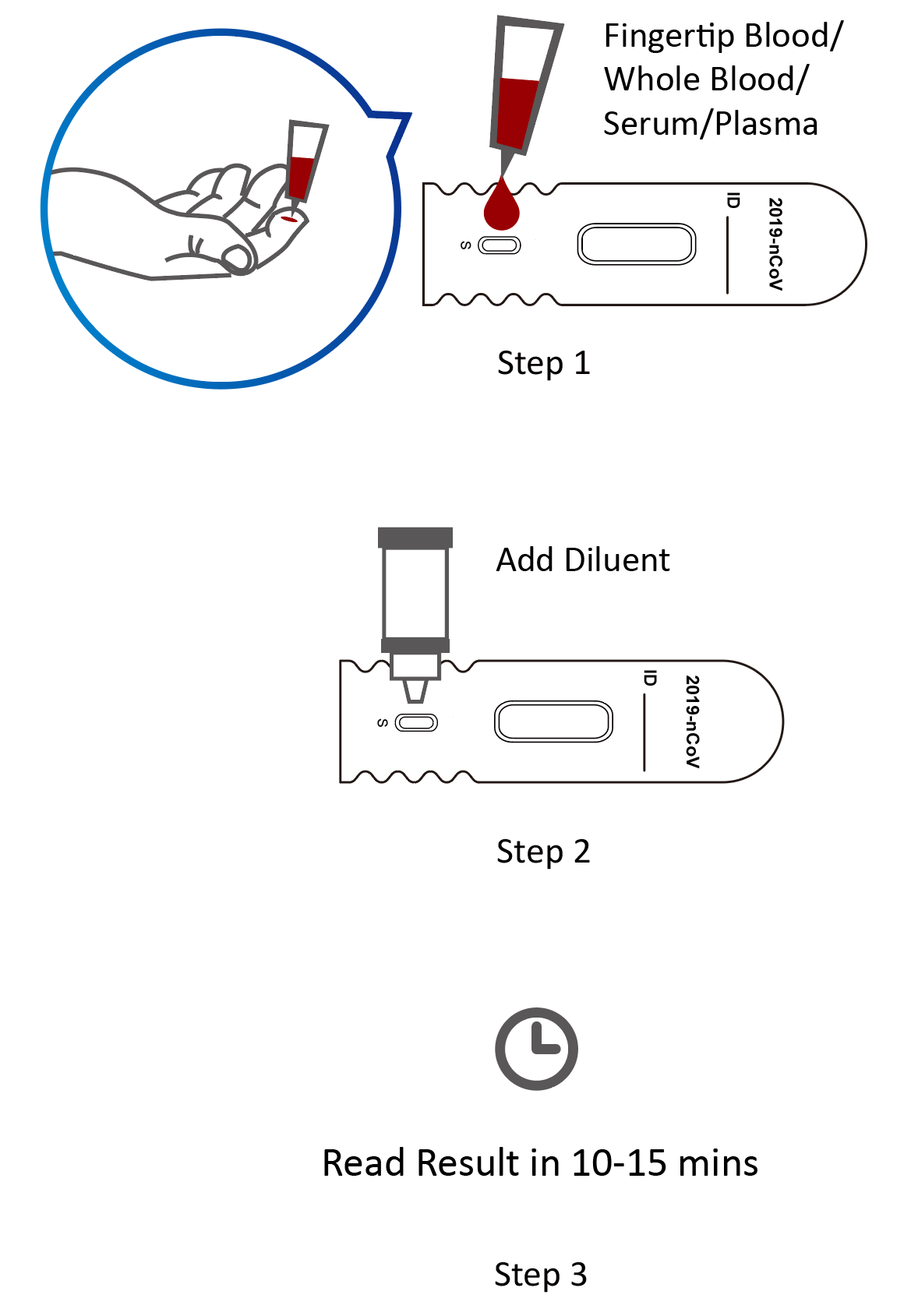
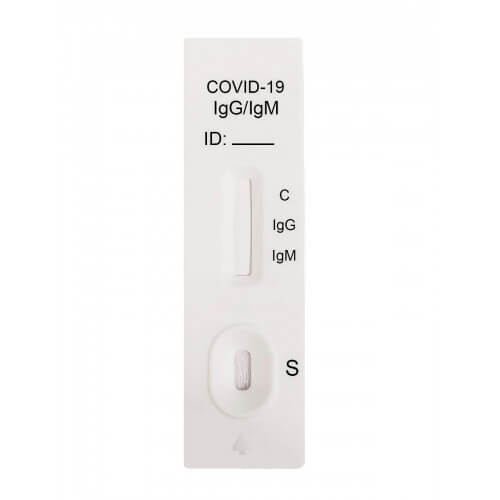
Rapid Diagnostic Test Covid 19 Instructions
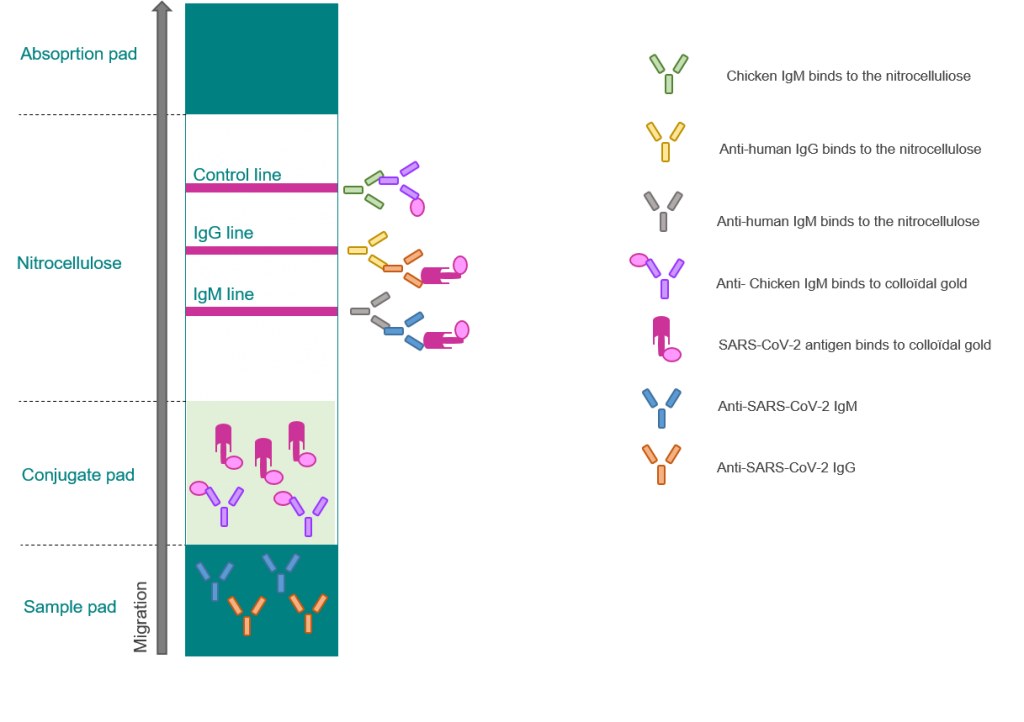
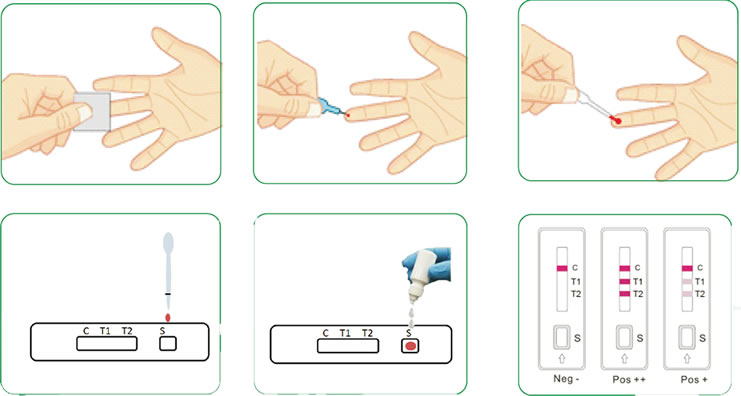
The covid 19 rapid poc ce ivd test is a lateral flow immunoassay.
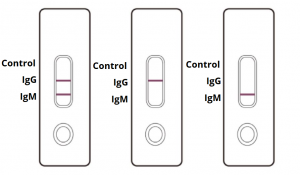
Rapid diagnostic test covid 19 instructions. A clia certified laboratory or testing site must report rapid antigen diagnostic test results to the local state tribal or territory health department in accordance with public law 116 136 18115 a the coronavirus aid relief and economic security cares act which requires every laboratory that performs or analyzes a test that is intended to detect sars cov 2 or to diagnose a. The covid 19 igm igg antibody rapid test is a qualitative test for covid 19 igm and igg antibodies to. Covid 19 testing methods steps and biological principles are explained in detail. Most kits include both igm and igg so.
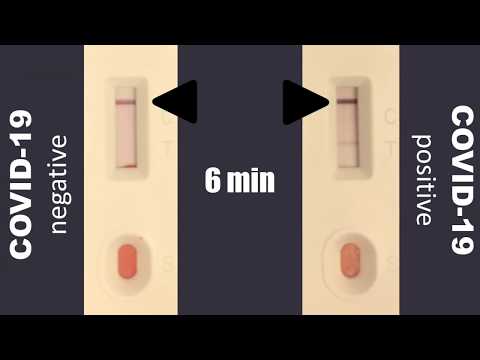
Complete instructions in english are included in the kit. Use of rapid antibody tests is contingent upon. 360bbb 3 b 1 unless the authorization is terminated or revoked sooner. Results may be available in minutes if analyzed onsite or a few days or longer in locations with test processing delays if sent to an outside lab.
Binaxnowtm covid 19 ag card is a rapid lateral flow immunoassay for the qualitative detection and diagnosis of sars cov 2 directly from nasal swabs without viral transport media. Also called a molecular test a health care worker collects fluid from a nasal or throat swab or from saliva. The results for this nucleic acid test might take up to 3 5 hours and the increased complexity of viral rna extractions and pcr reaction setups require highly trained laboratory personnel. For igm and 21 days i e.
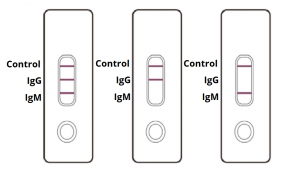
Therefore testing of covid 19 igm and igg antibodies is an effective method for the rapid diagnosis of covid 19 infection. Furthermore detection of covid 19 igm antibodies tends to indicate a recent exposure to covid 19 whereas detection of covid 19 igg antibodies indicates a later stage of infection. This is a rapid test for covid 19 and it will determine if you are contaminated with covid 19 or you have already suffered from covid 19 in the past based on igg and igm antibodies. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and or diagnosis of covid 19 under section 564 b 1 of the act 21 u s c.
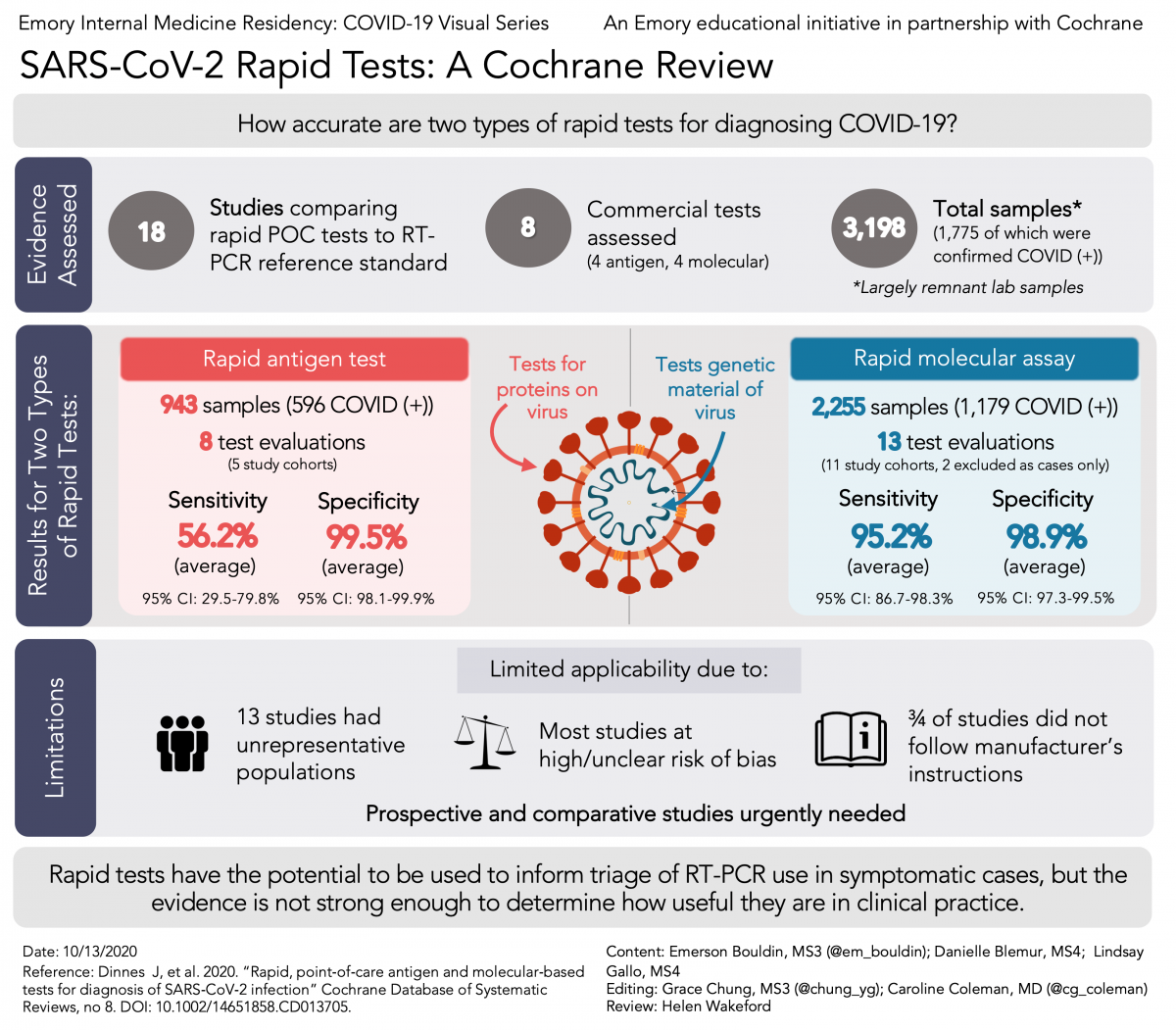
This covid 19 test detects genetic material of the virus using a lab technique called polymerase chain reaction pcr. Rapid covid 19 igg igm testing guidance consistent with fda guidance docket fda 2020 d 0987 the state of delaware has identified point of care lateral flow immunoassays rapid antibody tests as useful diagnostic adjuncts for covid 19 and subsequently developed guidance for use of these tests. The covid 19 rapid diagnostic test rdt can only be used on people who had onset of symptoms for at least 5 days i e.